Biomarkers: An Invaluable Tool for Therapeutic Drug Monitoring
To assess the safety and efficacy of a specific drug or medication, therapeutic drug monitoring (TDM) is used in a variety of clinical settings. The goal of TDM is to facilitate the individualization of therapeutic regimens, delivering safe and efficacious therapies to patients with optimum benefit.
In occurrences where the plasma level of a drug and its clinical effect are closely related, therapeutic drug monitoring is a powerful tool for managing effective patient care. Furthermore, in cases where a precise therapeutic end point is difficult to define, monitoring drug levels with TDM can provide valuable insight and aid in the determination of correct dosage regimens. The criteria for TDM include:
- Drugs with a narrow therapeutic index (therapeutic range)
- Long-term therapies
- Therapies which have a correlation between clinical response and serum concentration
- Drugs with variable pharmacokinetics
- Drugs that do not have a suitable biomarker associated with the desired therapeutic outcome
- Therapies which are co-administered with potentially interacting drugs
A variety of samples, including plasma, serum, whole blood, and dried bloodspots, can be used for therapeutic drug monitoring.
Drugs commonly monitored using therapeutic drug monitoring
Several drugs are commonly monitored using therapeutic drug monitoring, including:
- Antiepileptics
- Antiarrhythmics
- Antibiotics
- Antidepressants
- Antineoplastics
- Bronchodilators
- Cancer chemotherapy
- Cardio active drugs
- Immunosuppressives
Therapeutic concentration and therapeutic range/therapeutic window
For drugs with a narrow therapeutic range or therapeutic window, as those drugs are only effective over a small range of dosages, TDM is particularly helpful in managing patient care and dosage regimens. Additionally, not all patients have the same response at similar doses, thus adding still more complexity. In optimal dosing regimens, the blood concentration should not fall below the minimum effective concentration (MEC) and the peak blood concentration should not exceed the maximum safe concentration (MSC), as illustrated in Figure 1. A risk of treatment failure is present if a patient is under-dosed, and their concentration primarily remains at the subtherapeutic level. On the other hand, there is a risk of toxicity to the patient if they are over-dosed and the concentration is primarily above the target range.
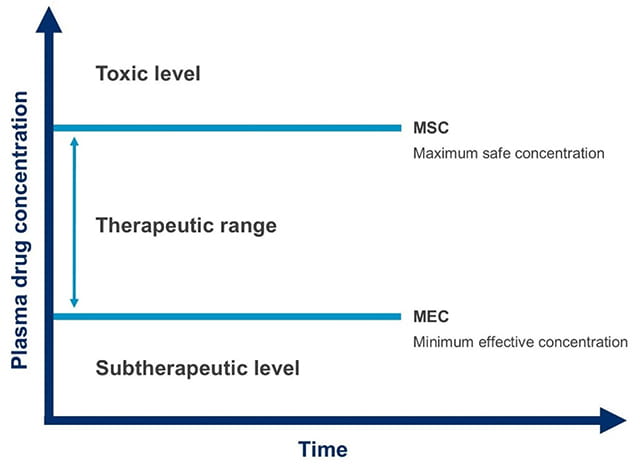
Figure 1. Therapeutic range (also known as therapeutic window), the concentration range of drug in plasma where the drug is efficacious without causing toxic effects in the majority of patients
The importance of PK/PD and ADME assays in therapeutic drug monitoring
Although the therapeutic range/window provides valuable insight into plasma drug concentration, target concentration is directly linked to individual dosage regimens, rather than a range of doses as shown in the therapeutic range graph (Figure 1). The concentration and effect relationship must be well-understood to accurately evaluate both desired and unfavorable effects. This understanding then enables the selection of an appropriate target concentration. Pharmacokinetics (PK) and pharmacodynamics (PD) help clinicians to understand how the body reacts to the drug, providing insight into pharmacological effects as well as therapeutic effects and outcome, as shown in Figure 2. In particular, five specific pharmacokinetic parameters are essential to therapeutic drug monitoring:
- Bioavailability
- Volume of distribution and distribution phases
- Clearance
- Half-life
- Protein binding of drugs
To summarize, PK/PD studies help to inform the target concentration, optimizing the balance between desired and unfavorable effects. Moreover, absorption, distribution, metabolism, and excretion (ADME) assays help to measure adherence, absorption, distribution, metabolism, and elimination of drugs in patients.
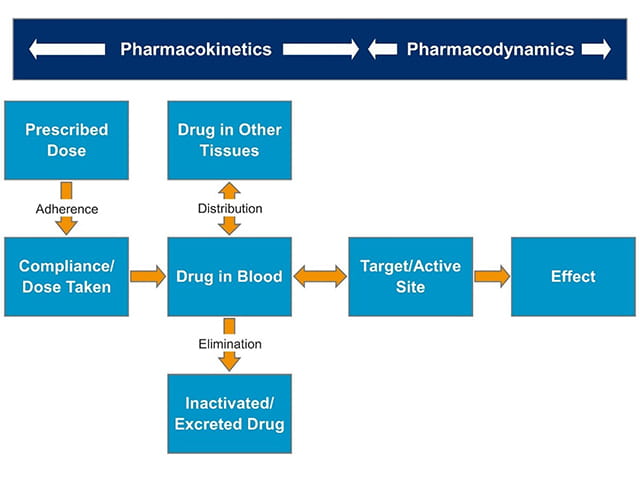
Figure 2. Processes involved in drug handling and therapeutic drug monitoring
Finding the right lab partner for therapeutic drug monitoring studies
Fundamentally, TDM is a multidisciplinary function that requires collaboration and effective, open communication between scientists, clinicians, nurses, and pharmacologists. In order to obtain actionable results and data, identifying the right scientific partner and lab for therapeutic drug monitoring studies is critical. Element’s consultative teams of scientists have a deep bench of knowledge and expertise in biomarker assay development, PK/PD, DMPK, and ADME assays. Our flexible teams can meet your needed turnaround time, delivering quality results on tight timelines, and our dedicated team of Project Managers facilitate consistent, open communication between clients and scientists. Partner with Element today for therapeutic drug monitoring to:
- Maximize therapeutic efficacy
- Identify therapeutic failure
- Facilitate dosage adjustments
- Facilitate the therapeutic effect of drugs by achieving target concentration
- Identify drug toxicity and abuse
Find related Resources
Learn more

Biomarkers 101: Intro to Biomarker Analysis
Selecting the right bioanalytical partner is just as critical as biomarker identification. Element's experts provide comprehensive bioanalytical support.

Bioanalysis Support
Element’s comprehensive bioanalytical testing services are dedicated to bringing industry-leading scientific expertise to help customers advance drug development programs.

DMPK & ADME Services
Element offers a wide range of ADME and DMPK laboratory services, and we can accept both in vivo and in vitro samples.
Sign Up for Free Resources
Visit Element's email subscription center to receive the latest industry news, technical whitepapers, case studies, webinars, and upcoming events.