Clinical Validation Testing
Accelerate your path to market and deliver life-changing medical devices to patients faster with turn-key physiological monitoring studies. Our comprehensive clinical validation streamlines your regulatory submission while optimizing device performance, helping you bring innovative health technologies to those who need them most.

What is Clinical Validation Testing at Element?
Clinical validation testing verifies the accuracy, safety, and effectiveness of medical devices through controlled human studies. At Element, we conduct physiological monitoring studies across multiple modalities, gathering the data needed for both product optimization and regulatory submission.

What Can Element Offer You For Clinical Validation Testing?
Components and materials we test
Components and materials we test
Our validation studies support your entire product lifecycle, covering pulse oximetry, blood pressure, temperature monitoring, respiratory rate, ECG, heart rate variability, sleep studies, cardiac output, EtCO2, and hemodynamic monitoring. With medical technology moving toward in-home monitoring, we validate the full spectrum of monitoring technologes from clinical-grade equipment to consumer wearables.
Key tests offered
Key tests offered
Your comprehensive validation package includes protocol design, IRB submission management, subject recruitment and compensation, data collection, and regulatory submission preparation. We deliver both formative and summative validation testing, with specialized expertise in human factors evaluation for clinical and home environments.
Our tests include:
- Blood Pressure Monitor Clinical Validation
- Respiratory Rate Monitor Validation Testing
- Temperature Monitoring Device Clinical Validation
- Pulse Oximetry Validation
- Heart Rate Monitor Device Validation
- Sleep Monitoring Device Validation
- Consumer Devices and Wellness Products
- Human Factors Testing
Methods and solutions offered
Methods and solutions offered
Real-world clinical testing combines with expert data analysis to optimize your device performance. Our staff specialists, including anesthesiologists, pulmonologists, and cardiologists, guide protocol development and execution, identifying opportunities for improvement throughout your development cycle. Real-time data collection enables your engineers to optimize and adjust algorithms based on actual user interaction.
- Blood Pressure Monitor Clinical Validation – Invasive arterial and non-invasive blood pressure testing follows the requirements of the current version of ISO 81060-2 and IEEE 1708, as well as the FDA consensus standard for blood pressure to the extent of recognition.
- Respiratory Rate Monitor Validation Testing – Respiratory rate accuracy performance of the test monitor is evaluated in highly controlled conditions over a range of respiratory rates in specific demographics.
- Temperature Monitoring Device Clinical Validation – Establish and execute a clear study plan for temperature monitor device validation testing.
- Pulse Oximetry Validation – Under highly controlled conditions, a test pulse oximeter will be evaluated for SpO2 accuracy performance over the specified range of oxygen saturation.
- Heart Rate Monitor Device Validation – Meet regulatory requirements for accuracy testing of heart rate monitoring devices with FDA-cleared ECG simulator or FDA-cleared ECG monitor human validation testing.
- Sleep Monitoring Device Validation - Ensure the safety of devices used in sleep studies to diagnose common sleep disorders.
- Consumer Devices and Wellness Products - Ensure user safety and support marketing claims for health monitoring devices sold as consumer products or consumer devices with certification, clinical validation, and usability testing.
- Human Factors Testing - Place devices in the hands of the user in a clinical or home environment to simulate actual use and discover any risks associated with use.
Which labs offer this service
Which labs offer this service
Access our full equipped primary laboratory at the Avista Hospital Medical campus near Denver, Colorado, plus our extensive network of medical centers and hospital research partners nationwide. This strategic positioning provides immediate access to clinical environments and diverse patient populations.
Regulatory pathway excellence
Regulatory pathway excellence
For more than three decades, our recognized industry experts guide your entire validation journey, from protocol development through successful submission. Get direct support during regulatory discussions and benefit from authors of multiple monitoring equipment guidance documents shaping your validation strategy.
Standards we test to and products we test
- ISO 81060-2
- IEEE 1708
- FDA consensus standard for blood pressure
Medical Monitoring Devices
- Blood pressure monitors (Invasive arterial and non-invasive)
- Respiratory rate monitors
- Temperature monitoring devices
- Pulse oximeters
- Heart rate monitors
- Sleep monitoring devices
Consumer Health Products
- Wearable health monitors
- Consumer temperature devices
- Heart rate tracking devices
- Sleep tracking products
Your Challenges, Our Solutions
Managing multiple testing partners
Navigating complex requirements
Making informed, quick decisions in R&D
Time to market pressures
Why Choose Element

Complete validation partner
Expertly Managed Clinical Validations
Unparalleled Regulatory and Medical Expertise
Optimize & Adjust Algorithms Throughout R&D
Related services

Heart Rate Monitor Device Validation
Element provides clinical validation testing for heart rate monitors using human and simulator data, meeting regulatory standards while delivering accurate performance. Their expert guidance addresses key accuracy challenges.

Human Factors and Usability Testing
Element's human factors and usability testing simulates real-world device use to reveal user errors and optimize performance. The service supports FDA guidelines and enhances overall device safety and effectiveness.
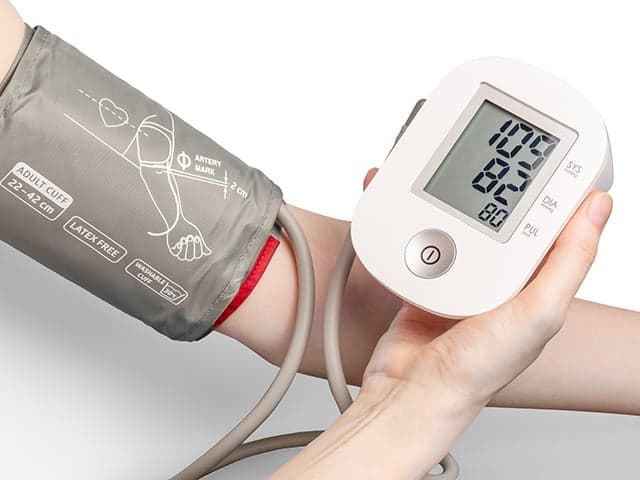
Blood Pressure Monitor Clinical Validation Testing
Element provides comprehensive blood pressure monitor clinical validation testing—from protocol design and participant recruitment to data analysis—leveraging invasive and non-invasive methods to overcome regulatory challenges and deliver precise device performance.

Pulse Oximeter Validation Testing
Element's pulse oximeter clinical validation service provides comprehensive human testing that delivers real-world, high-quality data to support regulatory submissions and significantly optimize device performance across diverse clinical settings with precision.
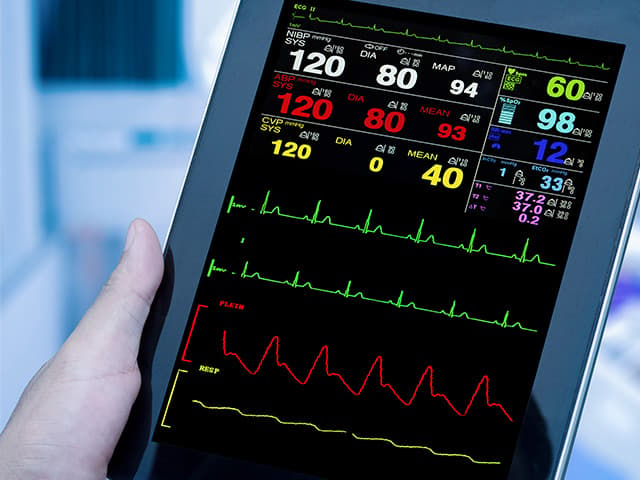
Respiratory Rate Monitor Clinical Validation Testing
Element offers expert-led respiratory rate monitor clinical validation testing, covering protocol design, participant recruitment, and regulatory-ready reports for streamlined submissions.

Sleep Monitoring Device Validation
Element provides clinical validation testing for sleep monitoring devices, supporting regulatory submissions and optimising device safety through expert-led data analysis and participant studies.

Temperature Monitoring Device Clinical Validation Testing
Element offers expert clinical validation testing for temperature monitoring devices, supporting regulatory requirements and fast-tracking your product to market with comprehensive study management.

Medical Device Regulatory Services
Element offers expert medical device regulatory consulting, guiding you through design, risk analysis, and FDA submissions for efficient product approval.

Consumer Electronics Testing and Certification
Get expert consumer electronics testing to accelerate your market entry. Element's accredited EMC, safety & wireless compliance services facilitate smoother market entry.

Battery Safety Testing for Medical Devices
Battery safety testing for medical devices, assessing performance, reliability, and compliance with international standards to support safe use in healthcare settings.

Medical Device Testing Services
With Element as your medical device testing partner, you’ll enjoy the benefit of a single comprehensive supplier across feasibility, R&D and prototype trials, through product development, regulatory validation and production quality control.

Medical Device EMC Testing
Element provides EMC testing and certification for Class I-III medical devices, helping manufacturers meet global regulatory standards and accelerate market entry with expert guidance and accredited laboratories.
- Heart Rate Monitor Device Validation
- Human Factors and Usability Testing
- Blood Pressure Monitor Clinical Validation Testing
- Pulse Oximeter Validation Testing
- Respiratory Rate Monitor Clinical Validation Testing
- Sleep Monitoring Device Validation
- Temperature Monitoring Device Clinical Validation Testing
- Medical Device Regulatory Services
- Consumer Electronics Testing and Certification
- Battery Safety Testing for Medical Devices
- Medical Device Testing Services
- Medical Device EMC Testing
